Research
Research Interests
My research interests are focused on electrochemical methods for energy storage and water treatment and corrosion testing and control. Additionally, my research interests include water and wastewaters treatment by different processes. I have published more than 70 papers in high impact factor international journals. My research areas cover the following topics:
- Electrochemical storage devices: Batteries, Fuel Cells, and supercapacitors
- Electrochemical technologies for water treatment
- Water and wastewaters treatment by chemical and physical processes
- Corrosion testing and control
Laboratory skills
- Chromatography techniques:
- HPLC and IC: High performance liquid chromatography and ion chromatography
- GC: Gas Chromatography
- GC/MS: Gas Chromatography coupled to mass spectrometry
- LC/MS/MS
- Atomic absorption and emission techniques
- Flame atomic absorption
- ICP and ICP-MS
- Electroanalytical methods:
- Polarography
- Voltammetry and potentiodynamic polarization
- Coulometry and electrolyses
- Amperometry
- Electrochemical impedance
- Routine chemical analyses:
- COD, TOC, TN, N-NH3, Ions (pH, Chlorides, fluorides, bromides, sulfates, nitrates, nitrites…)
Awarded Research projects
- QUCG-CAS- 22/23-602: High Performance Rechargeable Aqueous Zinc Ion Battery as a Potential Grid-Scale Energy Storage System
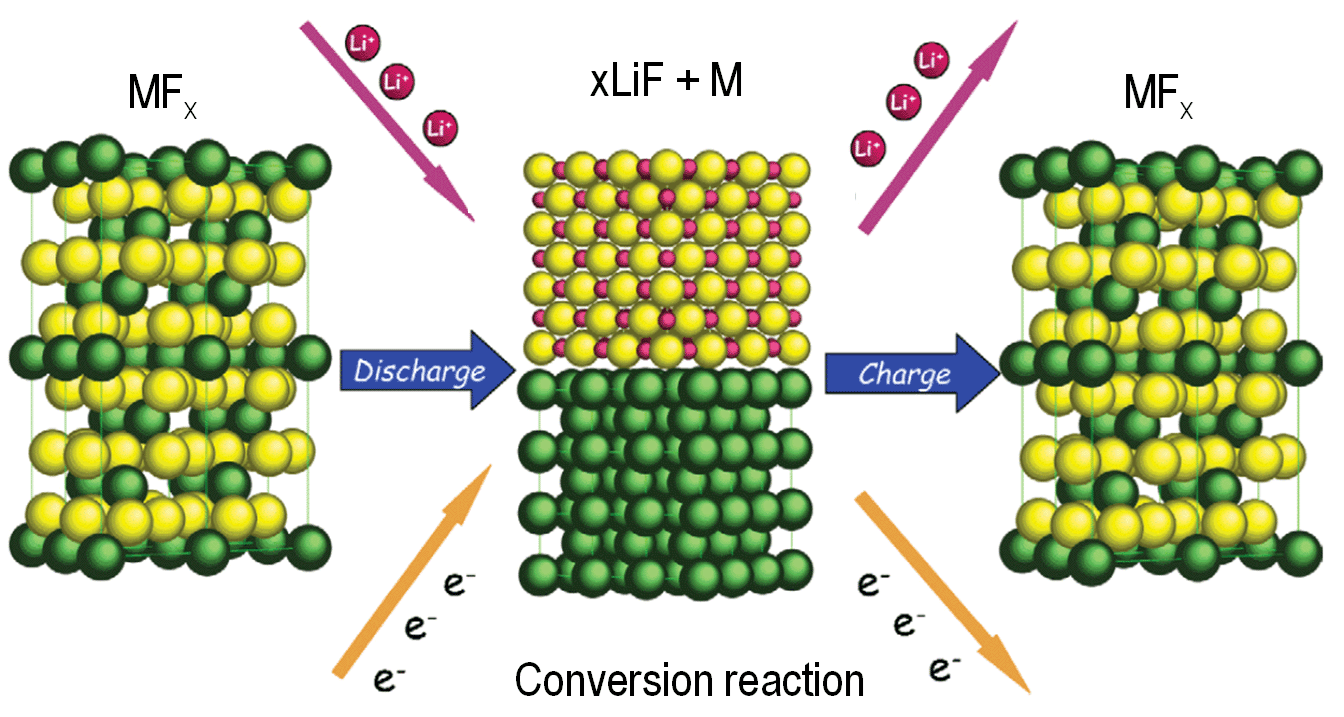
- QUCG-CAS- 20/21-4: Development of improved, super high energy, all-solid lithium-sulfur batteries
Fighting global warming for saving our planet requires with urgency, among other actions, the larger utilization of alternative energy resources and replacement of internal combustion car engines. All CO2-free energy sources would benefit from energy storage devices that could compensate time and space limitation of discontinuous energy production. The most attractive are portable electrochemical batteries and, in particular lithium batteries, due to their unique ability to deliver the stored chemical energy as electrical energy with high conversion efficiency and high energy density. Among these, a system that is attracting wide interest worldwide is the lithium-sulfur battery offering content in energy density orders of magnitude higher than that of the common lithium-ion batteries. However, the implementation of the Li/S battery has been so far hindered by a series of practical issues including: i) low electronic conductivity of both reactants and products; ii) solubility of polysulfide products into the electrolyte; iii) the reactivity of the lithium metal anode. Our strategy for addressing these issues will be that of completely renewing the Li/S battery chemistry; this including all its components. This strategy will allow us to improve consistently the performance of the Li/S battery upgrading it to a level of practical interest. Finally, this project is of strategic interest for Qatar where sulfur is largely available as by-product in gas processing plants.
- NPRP-7 – 567 – 2 – 216: Ternary Electrodes for Three Fold Increase in the Energy Density of Li-ion Batteries.
Lithium (Li)-ion batteries have proven to be vital in meeting the critical challenges of integrating renewable energy sources into a smart electrical grid as well as replacing internal combustion car engines with environmentally friendly electrical motors. To be cost-competitive, such applications require significantly reduced cost and increased energy density of the Li-ion cells beyond state of the art. The energy density of such cells depends on the volumetric capacity of their electrodes. Silicon (Si) – based anodes and fluoride (F) – based cathodes demonstrate great potential for the revolutionary enhancements in the energy storage of Li-ion cells. Unfortunately, these materials also suffer from several shortcomings, such as high electrical resistivity, low Li diffusivity and significant volume change during the battery operation, which limit their stability and power characteristics. By rationally nano-engineering the building blocks for these electrodes, we will overcome these limitations while delivering over 80 % of their theoretical capacity.
- Urine could be green energy and organic fertilizers
Urine can be considered as a special type of wastewater with very complex chemical composition. The urine is the fraction of domestic wastewater that contains the major part of plants nutrient with approximately 80% of nitrogen, 55% of phosphorus and 60% of potassium and very low metals content, being considered as natural resource of nutrients. However, the reuse of this wastewater as a fertilizer may result in transmission of pathogens. For this reason, it is necessary to treat the urine wastewater before any reuse. Urine could be used to generate electricity and as a free alternative to mineral fertilizers. Human urine together with wood ash could act as an alternative fertilizer for tomatoes. Equal amounts of tomato fruits are produced using urine/wood ash combination as mineral fertilized plants without posing any microbial or chemical risks. A technology has been developed to release hydrogen from ammonia compounds within urine. The hydrogen could be obtained from either human or animal urine. The gas can then be burned or used in fuel cells to power cars or electrical devices.
- Fulbright Scholarship in Research-University California, Irvine: Kinetic Models for the Free Radical Destruction of Micro-Constituents of Concern.
Recent reports have indicated that a surprisingly wide variety of pharmaceuticals and personal care products are entering the aquatic environment. Their bioactivity has been shown to be very potent at very low concentrations. Determining the extent and persistence of these compounds, micro-constituents of concern (MCOCs), has been a recent growth area in environmental chemistry. The growing group of compounds that are referred to as MCOCs has been a recent growth area in environmental chemistry. It is thought that radical chemistry is important in the fate and transport of many contaminants. Radiation chemistry makes it is possible to isolate reactions of various radicals with the chemicals of interest. Using reaction rates and steady state radical concentrations, pollutant lifetimes in aquatic systems can be estimated. Advanced oxidation processes use free radicals, principally hydroxyl radicals (.OH), which attack and decompose pollutants. For stable compounds, the free radical processes may be the principle pathway for degradation. An understanding of the kinetics involved and mechanistic details of the hydroxyl radical attack on organic compounds will aid in designing strategies for abating problematic environmental contaminants. Recent studies clearly show the need for a better mechanistic understanding as to the fate of these emerging pollutants of concern. The focus of this work was using radiation chemistry to study on the oxidation processes that will aide in mechanistic studies for MCOCs.
- Tunisian Chemistry Group grant: Purification of wet-process phosphoric acid by chemical and electrochemical technologies.
In this work, three technologies are studied for the purification of phosphoric acid produced by the wet process: chemical oxidation with hydrogen peroxide, adsorption onto activated carbon, and electrochemical oxidation by boron-doped diamond anodes. The treatment of wet-process phosphoric acid by chemical oxidation with H2O2 as oxidizing agent can remove 75 % of the initial TOC as maximum, indicating that this wet-process phosphoric acid contains an important amount of organics that cannot be oxidized by hydrogen peroxide under the operation conditions used. High temperatures and hydrogen peroxide/TOC ratios close to 150 g H2O2/g TOC allows obtaining the best chemical oxidation results. The adsorption onto activated carbon can remove between 40 and 60 % of the initial TOC as maximum. Adsorption times of 2 hours and activated carbon/WPA ratios close to 12 g AC/Kg WTP assures both steady state and maximum adsorption of organics. The electrochemical process is the only technique by which complete mineralization of WPA organics can be achieved. Operating at 60 mA cm–2 and at room temperature, high current efficiencies are achieved which only seem to decrease by mass transport limitations.
- Mobility travel grant Agence Universitaire de la Francophonie: Remediation of soils polluted with PAHs bty electrochemical technogies.
The coexistence of heavy metals and polycyclic aromatic hydrocarbons (PAHs) at many of the contaminated sites poses a severe threat to public health and the environment. Very few technologies, such as soil washing/flushing and stabilization/solidification, are available to remediate such sites; however, these technologies are ineffective and expensive to treat contaminants in low permeability clayey soils. Previous studies have shown that electrokinetic remediation has potential to remove heavy metals and organic compounds when they exist individually in clayey soils. In the present study, the feasibility of using surfactants and organic acids sequentially and vice versa during electrokinetic remediation was evaluated for the removal of both heavy metals and PAHs from soils. In this context, the goal of this work is to study the electrokinetic treatment of a natural soil polluted with phenanthrene (500 mg PHE kg−1 soil). The electrodes were positioned into semipermeable electrolyte wells with a linear distribution (two rows of three facing electrodes). Both electrolyte wells and the soil were in direct contact with the atmosphere because the electrokinetic pilot plant was an open system. To increase the solubility of phenanthrene and thus to enhance its transport through the soil, aqueous solutions of the anionic surfactant dodecyl sulfate (10 g dm−3) were used as a flushing fluid.
- Agencia Espanola de Coperaccion Internacional grant (34/04/P/E): Electrochemical treatment of organic pollutants in water
Surfactants are widely used in industry to promote the dispersion of organic species in water. Thus, they are widely used in the formulation of soaps, detergents, inks, etc. They normally consist of large molecules with both, hydrophobic and hydrophilic groups. They are highly soluble in water and persistent, once discharged into a natural environment. Thus, its study is interesting not only for being possible pollutants of industrial effluents but also because they are good models of complex pollutants. In thiswork, the electrochemical oxidation on boron-doped diamond of syntheticwastes polluted with surfactant sodium dodecylbenzenesulfonate (SDBS) has been studied. Results show that SDBS can be successfully removed with this technology inside different current densities and concentration ranges. The oxidation of the SDBS seems to occur in two main sequential steps: the first is the rapid degradation of SDBS, and the final is the less efficient oxidation of aliphatic intermediates to carbon dioxide. The nature of supporting electrolyte (NaCl, Na2SO4 and K3PO4) influences on the efficiency of the electrochemical oxidation process. The treatment of the NaCl solution seems to be more efficient in the chemical oxygen demand (COD) removal, while the sulphate and specially the phosphate media improve the TOC removal. However, in spite of this observation, chemical oxidation of SDBS by different types of oxidants cannot explain alone the results of the electrochemical oxidation with diamond anodes. This suggests that the synergistic effect of the different oxidation mechanisms that occurs into the electrochemical cell (direct oxidation and mediated oxidation by hydroxyl radicals and by oxidants formed from the electrolyte) is the responsible of the great efficiencies obtained with this technology in the treatment of organics.
4- On going/New Research Projects
- Development of improved, super high energy, all-solid lithium-sulfur batteries
Fighting global warming for saving our planet requires with urgency, among other actions, the larger utilization of alternative energy resources and replacement of internal combustion car engines. All CO2-free energy sources would benefit from energy storage devices that could compensate time and space limitation of discontinuous energy production. The most attractive are portable electrochemical batteries and, in particular lithium batteries, due to their unique ability to deliver the stored chemical energy as electrical energy with high conversion efficiency and high energy density. Among these, a system that is attracting wide interest worldwide is the lithium-sulfur battery offering content in energy density orders of magnitude higher than that of the common lithium-ion batteries. However, the implementation of the Li/S battery has been so far hindered by a series of practical issues including: i) low electronic conductivity of both reactants and products; ii) solubility of polysulfide products into the electrolyte; iii) the reactivity of the lithium metal anode. Our strategy for addressing these issues will be that of completely renewing the Li/S battery chemistry; this including all its components. This strategy will allow us to improve consistently the performance of the Li/S battery upgrading it to a level of practical interest. Finally, this project is of strategic interest for Qatar where sulfur is largely available as by-product in gas processing plants.
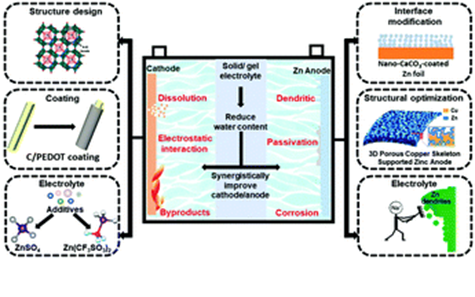
- High Performance Rechargeable Aqueous Zinc Ion Battery as a Potential Grid-Scale Energy Storage System
The purpose of this project is to develop long-life, high-power aqueous zinc-ion batteries (AZIBs) with rechargeability as well as to understand their operation mechanisms. The developed AZIBs will be targeted to grid-scale energy storage systems (ESSs) that are essential for maintaining and distributing energy generated from various sources in the near future. In contrast with current commercial lithium-ion batteries (LIBs), AZIBs can offer advantages in terms of high safety, low cost, fast kinetics, etc. Toward this end, both the cathode and anode will be optimized and studied separately. For the cathode, we will focus on materials that function based on hydrated intercalation and improve them for robust and fast operation. The main reason for our interest in materials capable of hydrated intercalation or with crystal water is based on our previous research experience that reveals that hydrated materials enable fast intercalation kinetics and structural stability over long-term cycling. The role of crystal water will be investigated in-depth using various analytical tools and density functional theory (DFT) calculations. Accordingly, materials will be designed in consideration of the role of crystal water. Another critical concern lies with the anode. The use of Zn metal inevitably leads to chronic issues such as hydrogen evolution, corrosion and dendrite formation. These degradation processes lower the usable portion of the Zn metal anode and ultimately lower the overall energy density of a cell since excess Zn metal is needed 4. In an effort to address such issues with the Zn metal anode, we plan to undertake various approaches: 1) implementation of an ionically conductive protection layer, 2) integration of a water-in-ionomer electrolyte, and 3) optimization of salt conditions. Once the cathode and anode designs are identified, both electrodes will be paired and optimized further from the perspective of maximizing full-cell performance. In pursuit of applying to on-site ESS sectors, finally, cell performance, cost, scale-up, and maintenance will be all taken into consideration under communication with battery manufacturers who have had a longstanding collaboration with the Korean team. The development of a new energy storage paradigm based on AZIBs would position Qatar as a key player by possessing the core know-how in material design and cell assembly.
- Energy production from sunlight using novel photovoltaic cells based on algae microbial fuel cells
Sun is the primary source of energy for Earth and a very important resource in Qatar and in many countries worldwide. For centuries, many attempts have been done to develop efficient technologies to deal with the production of electricity from it. This project deals with the development of a novel technology based on the synergy of the concepts of microbial fuel cells and photovoltaic cells in order to try to recover a significant amount of energy as electric energy from sunlight. The system consists of a microbial electrochemical cell in which an algae culture produces oxygen using solar energy in the cathodic compartment, and an organic substrate is oxidized by electricity-producing microorganisms in the anodic compartment. Although not necessary, this organic substrate could be a wastewater, giving an environmental added value to the device. The goal of the project is to develop a small prototype of this technology. To attain this goal, many activities dealing with a proper design of the electrochemical cell have to be done, including the assessment of electrode materials, the study of the separation between anodic and cathodic compartments, the selection of cathodic materials to let sunlight be used efficiently by algae, the methodology to develop and operate algae and electricity-producing microorganisms cultures, the influence of nutrients and organic substrate solutions, and the optimization of cell operation in order to get a robust technology.
- Conductive Polymer – Carbon Nanocomposites As a New Technology Platform for Capacitive Desalination
During the CDI process, the cations and anions from the salt solutions move to the surface of anode (negative electrode) and cathode (positive electrode), respectively, and adsorb on their surface under an application of a potential. Conventional electrodes in CDI are based on activated carbon electrodes, which offer limited ion storage capacitance per unit electrode (and device) volume. In addition, due to the presence of bottleneck pores in activated carbons and the torturous shape of their pores, the rate of the ion transport and thus the rate of the capacitive desalination is relatively slow. Finally, the limited strength of the conventional activated carbon-based CDI electrodes limits their cycle stability under operating conditions. Conductive polymers offer higher specific (mass normalized) and volumetric (volume-normalized) capacitances than activated carbons, but suffer from volume changes during ion adsorption/desorption, which commonly lead to their rapid degradation. Recent results, however, show that dramatic enhancements in the stability in conductive polymers could be achieved if thin layers of polymers are deposited on carbon fibers. Moreover, the produced composites demonstrate remarkable mechanical properties with tensile strength and modulus of toughness higher than that of aluminum matrix composites, titanium and aluminum alloys, steels and many other common structural materials. Our aim is to investigate a carbon – conductive polymer composite membranes for the CDI, which have the potential to revolutionize the CDI technology by enhancing both the ion removal capacity and the rate of the desalination.
- Development of New Innovative Aluminum-Air Batteries
The objective of this proposal is, to obtain a high power performance rechargeable battery by studying the fundamental electrochemistry of a metal-air battery that uses an oxygen ion conductive solid oxide electrolyte (SOE). This project deals with the development of a novel technology based on the synergy of the concepts of conventional metal-air batteries and solid oxide fuel cells (SOFCs) to provide a high-energy low cost storage system for utility applications. In this study, we will study the aluminum and air electrodes with the objective of decreasing the oxide layer that forms on the aluminum. Decreasing this oxide layer will maximize the available energy density at high power which are the requirements of a large scale low cost battery system.
- Effects of impurities on the current efficiency and metal quality in Aluminum smelters
Certain key impurity elements should be identified. These may be phosphorus, iron, silicon, sulfur and others. By getting access to data from routine analysis of produced aluminium over a rather long time period it would interesting to correlate the contents of impurities to operational changes including change of raw materials, startup period of new cells, anode effect and possibly changes in temperature, current load and current efficiency. The impurity levels of alumina and anode carbon should also be known. Also data from measurement campaigns (metal and electrolyte) could be interesting. Available analysis data of exit gas including particulates and carbon dust should be interesting too. Measurement campaigns may be carried out by additions of impurities to certain cells and analyzing samples of bath and metal as a function of time. The distribution of impurity elements in metal, electrolyte and gas may be determined by such experiments A comparison of impurity behavior between Qatalum and an aluminium plant in Norway may also be carried out. The current efficiency could be determined on a short term basis by using trace elements such as copper. The aim of the project is to quantify the effects of selected impurities on the current efficiency for aluminium production and the purity of the produced aluminium.
- Treatment of synthetic urine wastewaters by electrochemical oxidation using different anode materials.
In this work, the electrochemical oxidation of synthetic urine by anodic oxidation using boron-doped diamond (BDD) as anode and stainless steel as cathode was investigated. Results show that complete depletion of COD and TOC can be attained regardless of the current density applied in the range 20 – 100 mA cm-2. Oxalic and oxamic acids, and, in lower concentrations, creatol and guanidine were identified as the main intermediates. Chloride ions play a very important role as mediators and contribute not only to obtain a high efficiency in the removal of the organics but also to obtain an efficient removal of nitrogen by the transformation of the various raw nitrogen species into gaseous nitrogen through chloramine formation. Main drawback of the technology is the formation of chlorates and perchlorates as final chlorine products. The increase of current density from 20 mA cm-2 to 60 mA cm-2 led to an increase in the rate of COD and TOC removals although the process becomes less efficient in terms of energy consumption. The most efficient conditions are low current densities and high temperature reaching total mineralization at an applied charge as low as 20 kAh m-3. This result confirmed that the electrolysis using diamond anodes is a very interesting technology for the treatment of urine.