Pharmaceutical Industry

Results-driven pharmaceutical executive at Hikma Pharmaceuticals (FDA-approved), with a proven track record of bringing complex products to market, including the development and registration of 21 ANDA products for the US and MENA regions.
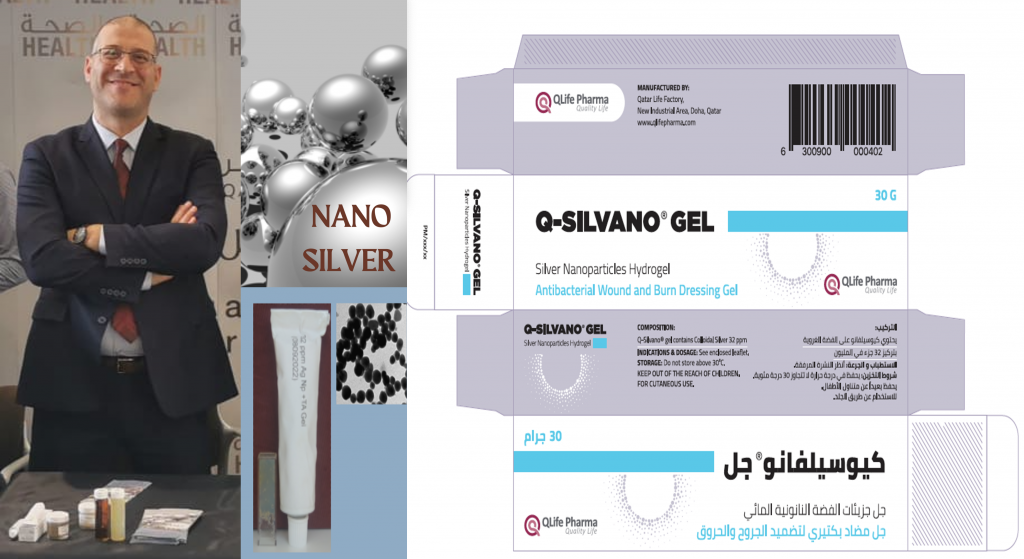
At Qatar University, led the innovation and regulatory strategy for Q-Silvano, the nation’s first registered nanotechnology-based medical product, translating cutting-edge research into real-world impact.
Key Achievements:
- Product Development & Commercialization:
- Led the end-to-end development, from pre-formulation to market launch, of 21 Abbreviated New Drug Applications (ANDA) for solid oral dosage forms, successfully registered and commercialized in the highly regulated US and MENA markets.
- Managed all aspects of product lifecycle, including formulation optimization, scale-up, technology transfer, and overseeing bioequivalence studies to ensure therapeutic equivalence.
- Pioneering Innovation:
- Conceived and executed the development of Q-Silvano, a groundbreaking nanotechnology-based medical device, navigating the full regulatory pathway to achieve the first official registration of its kind in Qatar.
- Regulatory & Quality Leadership:
- Ensured strict compliance with FDA, EMEA, and ICH guidelines across all projects, successfully passing regulatory inspections and mitigating compliance risk.
- Implemented robust Quality by Design (QbD) principles to establish proven acceptable ranges and ensure process validation and reproducibility.
| 2004-2006 | Formulation Manager (R&D) Hikma Pharmaceuticals (http://www.hikma.com) Amman-Jordan |
| 2000-2004 | Formulation Scientist (R&D) Hikma Pharmaceuticals (http://www.hikma.com) Amman-Jordan |
- Vast experience in the pharmaceutical industry and generic product development, covering pre-formulation, formulation, scale-up, and bio-equivalence trials within an FDA-approved pharmaceutical giant like Hikma Pharmaceuticals (http://www.hikma.com).
- Exceptional theoretical and practical proficiency across various pharmaceutical unit operations, including blending, granulation, drying, milling, compaction, tablet compression/coating, capsule filling, and thorough quality control evaluations.
- Proven track record in seamlessly transferring technology from R&D to production and between production sites, showcasing adeptness at maintaining operational continuity and efficiency.
- Expert command of international regulations such as GMP, GLP, GCP, FDA, EMEA, and ICH guidelines, underscoring a commitment to unparalleled standards of compliance and quality assurance.
